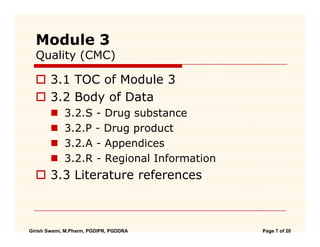
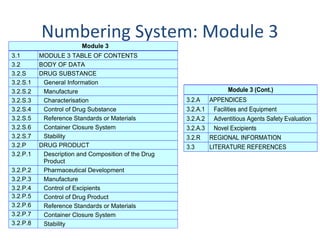
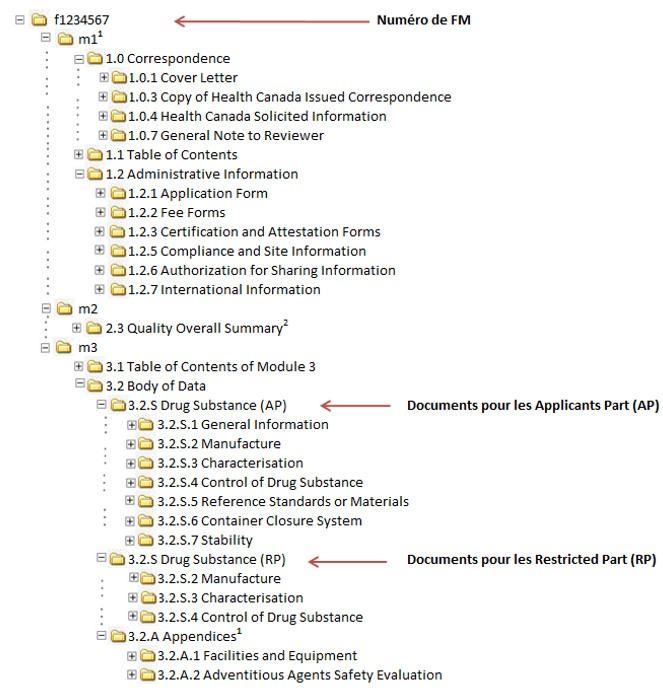
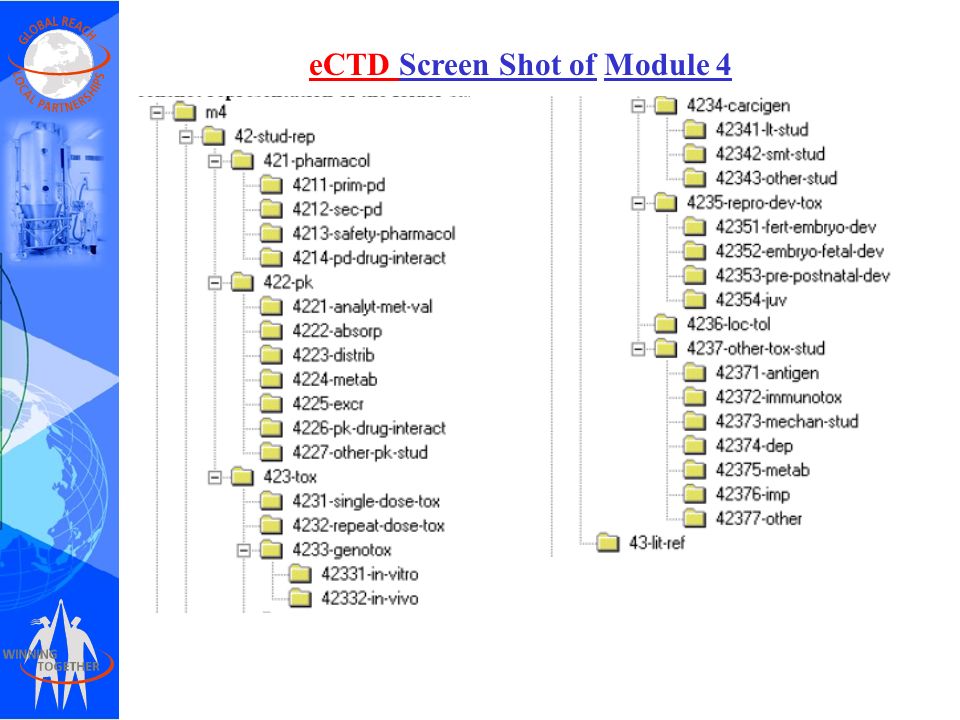
![PDF] Between Standardisation and Flexibility – Defining Granularity of the eCTD Module 3.2.S for Different Types of Drug Substances in Europe | Semantic Scholar PDF] Between Standardisation and Flexibility – Defining Granularity of the eCTD Module 3.2.S for Different Types of Drug Substances in Europe | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/30903f1fc51c4917a2877b9cf3756ccc7fc6425a/11-Table1-1.png)
PDF] Between Standardisation and Flexibility – Defining Granularity of the eCTD Module 3.2.S for Different Types of Drug Substances in Europe | Semantic Scholar

Drug Administration Bangladesh CTD Module 2017 FINAL | PDF | Medical Treatments | Medicinal Chemistry
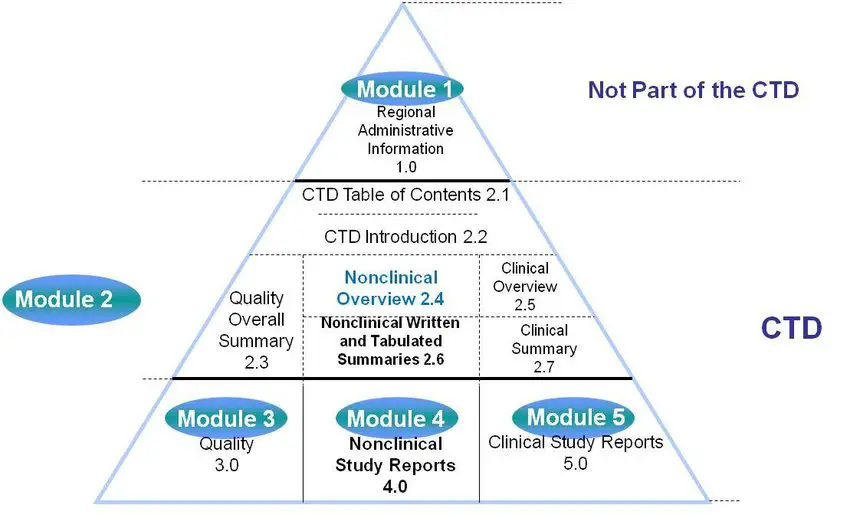
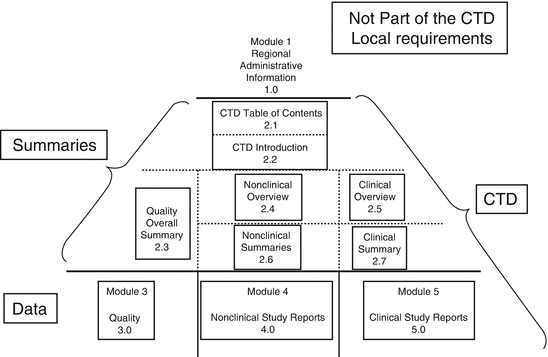
ELECTRONIC COMMON TECHNICAL DOCUMENT (eCTD): A REVIEW OF HISTORY, BENEFITS OF IMPLEMENTING, CHALLENGES, MODULES, RISKS INVOLVED

PDF) Manufacturing process development of ATMPs within a regulatory framework for EU clinical trial & marketing authorisation applications
![PDF] Between Standardisation and Flexibility – Defining Granularity of the eCTD Module 3.2.S for Different Types of Drug Substances in Europe | Semantic Scholar PDF] Between Standardisation and Flexibility – Defining Granularity of the eCTD Module 3.2.S for Different Types of Drug Substances in Europe | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/30903f1fc51c4917a2877b9cf3756ccc7fc6425a/8-Figure1-1.png)
PDF] Between Standardisation and Flexibility – Defining Granularity of the eCTD Module 3.2.S for Different Types of Drug Substances in Europe | Semantic Scholar
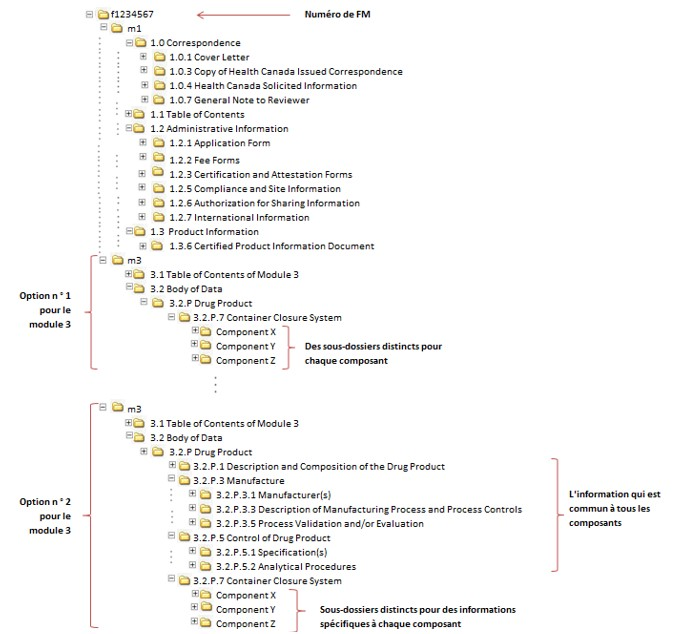
Between Standardisation and Flexibility – Defining Granularity of the eCTD Module 3.2.S for Different Types of Drug Substan